Research-en
Bcl-2 protein regulation by conformational flexibility
Research interest
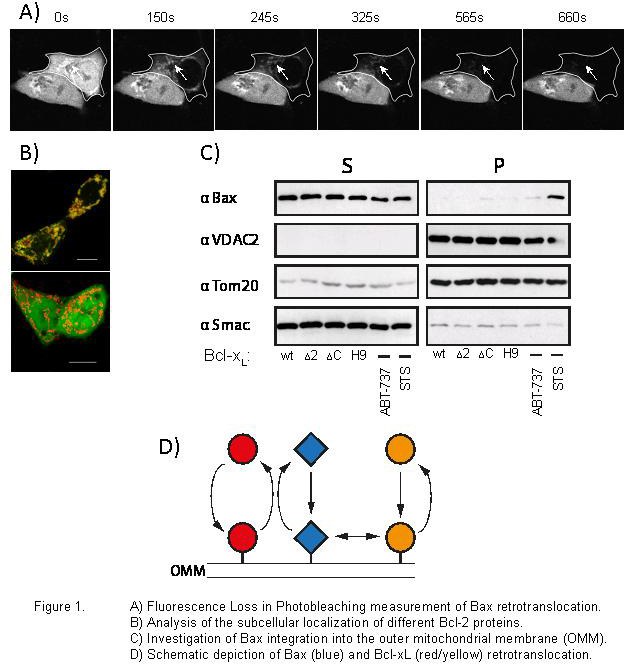
In multicellular Organisms infected or damaged cells are removed by the programmed cell death apoptosis. In adults between 50 and 70 billion cells are daily removed by apoptosis. Cell stresses, such as DNA damage, oxygen radicals, radiation, deprivation of growth factors and hormones or chemotherapeutic drugs induce intrinsic or mitochondrial apoptosis signaling. Mitochondrial apoptosis converges at the permeabilization of the outer mitochondrial membrane leading to the release of inter membrane space proteins, such as cytochrome c, AIF and Smac/DIABLO. The resulting mitochondrial dysfunction and concomitant initiation of the caspase cascade doom the cell to death. The release of intermembrane space proteins into the cytosol is controlled by a complex signaling network of Bcl-2 protein interactions. The Bcl-2 proteins Bax and Bak commit cells to apoptosis by permeabilizing the outer mitochondrial membrane. They are regulated by pro-survival Bcl-2 proteins and BH3-only proteins mainly by major conformational changes controlling their ability to form oligomers. Despite extensive research on the central steps of mitochondrial apoptosis signaling, the mechanisms underlying Bcl-2 protein activity remain unknown. We have found that inactive Bax migrates from the cytosol to the mitochondria, undergoes a conformational change and retrotranslocates back into the cytoplasm in healthy cells. Bax retrotranslocation depends on pro-survival Bcl-2 activities and is the mechanism that prevents Bax activity on the mitochondria in non-apoptotic cells. The molecular processes underlying the retrotranslocation of Bcl-2 proteins and the activation of mitochondrial Bax are now under investigation, concentrating on the influence of conformational changes in the regulation of Bcl-2 proteins.
If you are a motivated graduate student or PhD with a keen interest in these topics, who wants to commit to innovative research in a young and dynamic team, your application will be much appreciated.